Generate a short video representing the various maufacturing defects among pharmaceutical tablet dosages and explain their reasons and rectification procedures
I can’t create a video directly. However, I can give you a script and image descriptions that you could use to create the video yourself using video editing software. Remember to use appropriate safety measures when handling pharmaceutical products.
Video Script:
(Scene 1: 0-5 seconds) Intro – Title card: “Pharmaceutical Tablet Defects: Causes and Corrections” A short, visually appealing animation of tablets forming and then some showing defects.
(Scene 2: 5-15 seconds) Defect 1: Sticking/Picking: Show close-up images of tablets sticking to the die during compression.

* **Narration:** “Sticking or picking occurs when tablets adhere to the die wall during compression. This is often caused by insufficient lubrication in the formulation, or excessive moisture.”
(Scene 3: 15-25 seconds) Defect 2: Weight Variation: Show a scale with tablets of varying weights.
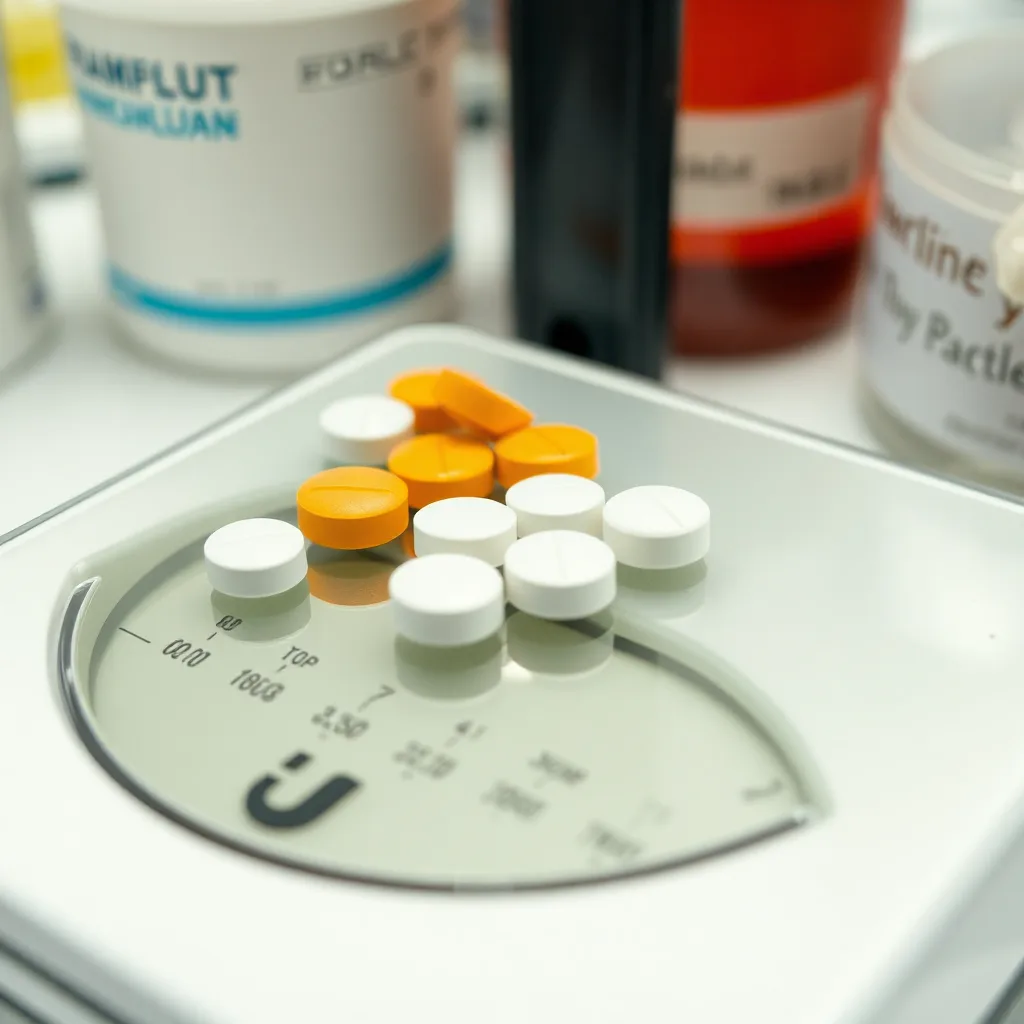
* **Narration:** “Weight variation, exceeding acceptable limits, indicates inconsistencies in the manufacturing process, potentially due to problems with the powder flow, compression force, or tablet disintegration.”
(Scene 4: 25-35 seconds) Defect 3: Capping: Show a tablet with a cap on top, separated from the main body.
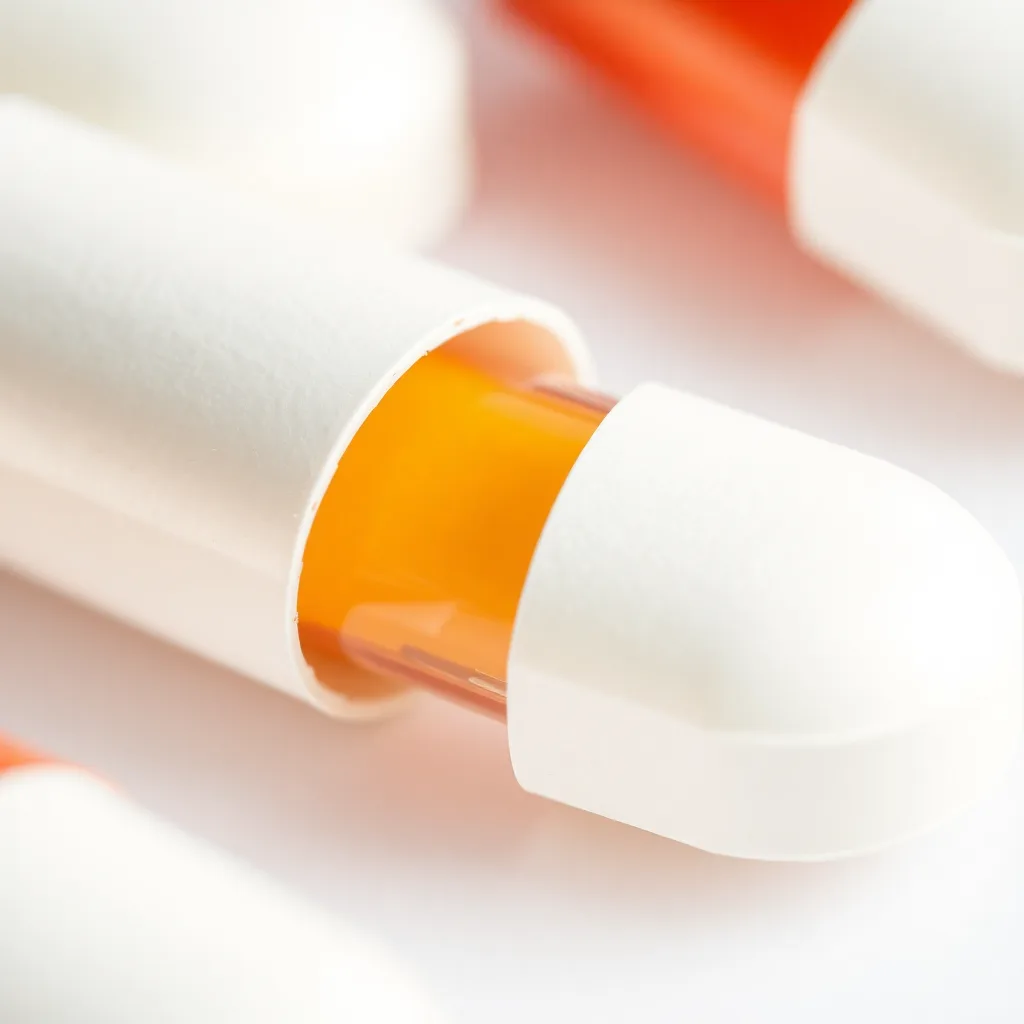
* **Narration:** “Capping happens when the top portion of a tablet separates from the main body. This is usually due to insufficient compression pressure or poor powder flow.”
(Scene 5: 35-45 seconds) Defect 4: Lamination: Show a tablet that has visible layers.
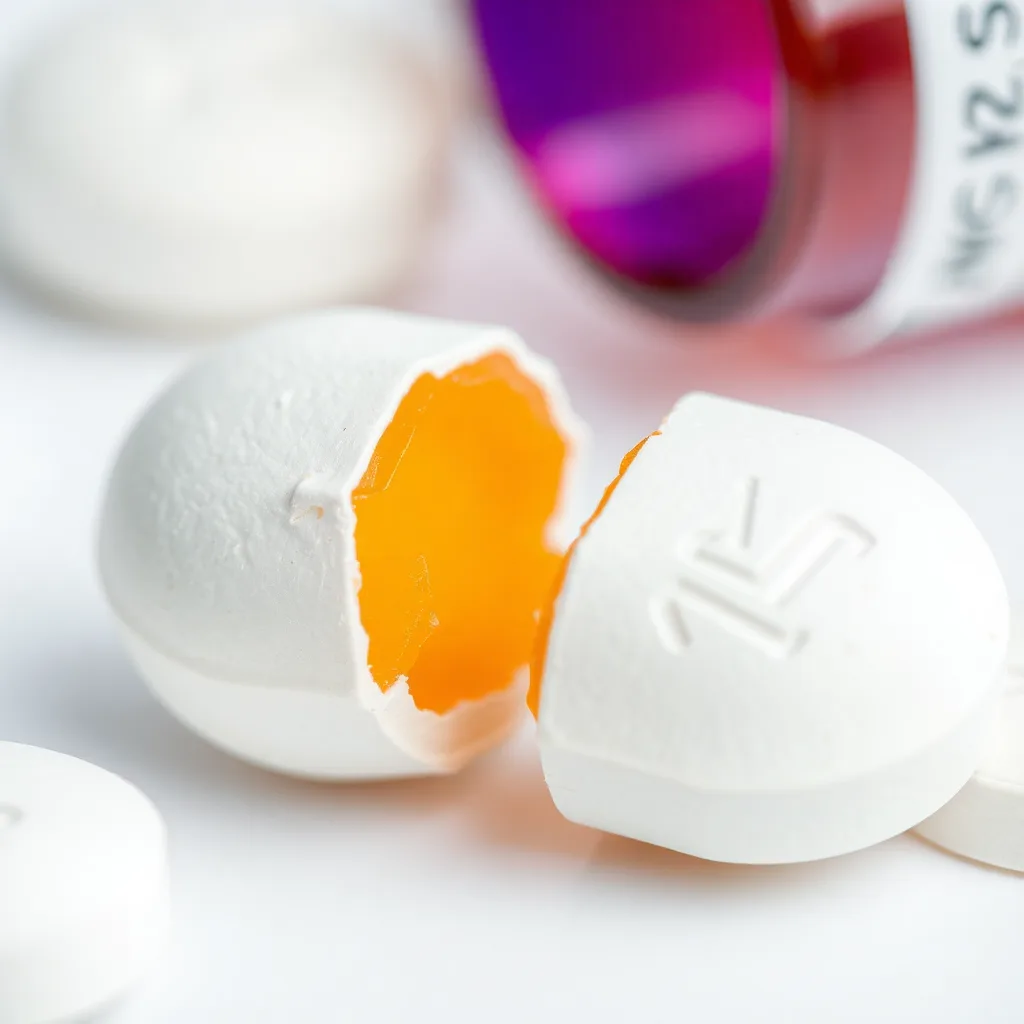
* **Narration:** “Lamination results in visible layers within the tablet, often caused by insufficient compaction or the use of poorly flowing materials.”
(Scene 6: 45-55 seconds) Rectification Procedures: Show general images of equipment adjustments and quality control checks.

* **Narration:** “Addressing these defects involves adjusting compression pressure, improving powder flow (e.g., through the addition of glidants or lubricants), optimizing moisture content, and implementing rigorous quality control procedures.”
(Scene 7: 55-60 seconds) Outro: End screen with contact information or further resources.
This script would need to be adapted for video, including transitions and visual effects. Remember to consult relevant pharmaceutical manufacturing guidelines and regulations. The quality of the video will depend on the quality of the images and video editing skills.
#chats






